Carbonic Acid Strong or Weak
Carbon dioxide and water react chemically to produce carbonic acid a weak acid thats been shown to stimulate the same nerve receptors in your mouth as mustard. AH H 2 O A-aq H 3 O aq.
What Are Some Examples Of The Most Commonly Used Weak Acids Quora
At equilibrium both the acid and the conjugate base are present in solution.

. All new derivatives S1S22 were assayed against human carbonic anhydrase hCAs IX and XII for their inhibitory. Fizzing occurs because of the production of the hydrogen gas obtained due to reaction of the acid on the magnesium ribbon. In biomedical sciences and is a science writer educator and consultant.
Hence the color of pH paper is blue. Acetic acid has a Ka value of 18 x 10-5. Electrochemical reduction of carbon dioxide CO 2 is a promising means of converting this greenhouse gas into valuable fuels and chemicalsHowever two competing reactions restrict the efficiency of this process.
Sodium hydroxide is now a strong base that is fully ionised and produces a significant number of hydroxide ions OH aq. A NaOH is a strong base. Therefore more fizzing take place in test tube A.
A The pH paper shows red colour for strong acids and blue colour for strong bases. Since the Ka is quite small acetic acid is a weak acid. Citric acid is a weak acid found in citrus fruits and used as a natural preservative and to impart a sour flavoring.
She has taught science courses at the high school college and graduate levels. The result is that water contains the weak acid carbonic acid. D pH of base is above 7 and higher than water or acid.
In base much of the CO 2 is trapped as carbonate before reduction. A weak acid or a weak base only partially dissociates. It is responsible for dissolving limestone to produce geological features such as stalagmites and stalactites.
CO 3 2-HCO 3-Hydrogen carbonate ion. Carbonic Acid H 2 CO 3 Nitrous Acid HNO 2 Hydrocyanic Acid HCN Hydrosulfuric Acid H 2 S Citric Acid C 6 H 8 O 7 List of Weak Bases. As explained earlier plant roots can grow in gaps beneath concrete and create a strong enough force to break it.
In this study new sulphamethoxazole derivatives S1S4 S6S12 and S14S22 were designed and synthesized and their structures were fully characterized and validated using NMR mass and IR spectroscopy as well as elemental analyses. Strong base SBA Type 1. A strong base is a basic chemical compound that can remove a proton H from or deprotonate a molecule of even a very weak acid such as water in an acidbase reaction.
An acid which is completely ionised in water and produces H is called Strong Acid. Atoms are represented as spheres and are colour-coded. This carbon dioxide CO 2 when combined with water H 2 O produces weak carbonic acid which can degrade the surfaces of rocks and rock particles.
C Strong acid is red neutral solution is green and strong base is blue with pH paper. The most strongly basic functional group available with the greatest affinity for the weak acids such as silicic acid and carbonic acid that are commonly present during a water demineralization process. But so far the evidence for harm doesnt seem to be very.
Acetic acid CH 3 COOH Carbonic. The efficiency of regeneration of the resin to the hydroxide form is somewhat lower than Type 2. As a result the aqueous solution is pH neutral.
If you gulp it down it can of course give you hiccups or indigestion. Strong acids are listed at the top left hand corner of the table and have Ka values 1 2. The strong bases are listed at the bottom right of the table and get weaker as we.
Acid with values less than one are considered weak. Examples of strong acids and bases are given in the table below. A NaHCO 3 is a strong base.
Since HCl is a very strong acid there is a lot of liberation of hydrogen gas from test tube A. HCl is a strong acid whereas acetic is a weaker acid. Common examples of strong bases include hydroxides of alkali metals and alkaline earth metals like NaOH and CaOH 2 respectively.
Hydrochloric acid HCl Sulphuric acid H 2 SO 4 Nitric acid HNO 3 Weak Acids An acid which is partially ionised in water and thus produces a small amount of hydrogen ions H is called a Weak Acid. In aqueous solution each of these essentially ionizes 100. Carbonic acid is a weak acid.
Carbonic acid on the other hand is a weak acid. On the other hand a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. In acid protons outpace CO 2 at catching electrons from the cathode.
Helmenstine holds a PhD. Sometimes the effect can be damaging. A conjugate acid within the BrønstedLowry acidbase theory is a chemical compound formed when an acid donates a proton H to a basein other words it is a base with a hydrogen ion added to it as in the reverse reaction it loses a hydrogen ion.
When sodium carbonate is dissolved in water it is partially hydrolyzed yielding sodium hydroxide and carbonic acid. Example Is Acetic Acid Strong or Weak.
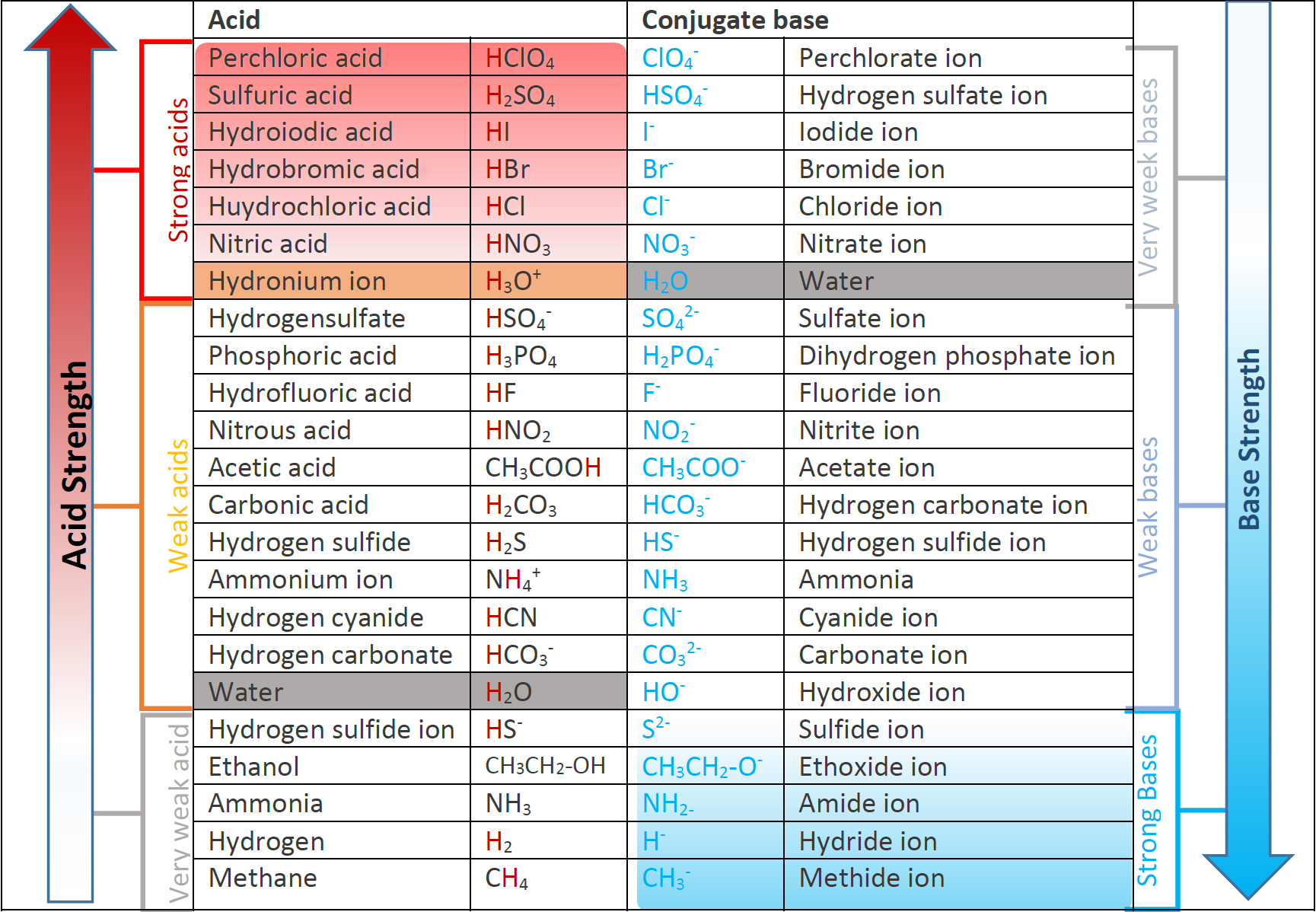
6 3 Strength Of Acids And Bases Chemistry Libretexts

Is Carbonic Acid Is A Strong Electrolyte Quora

Is H2co3 An Acid Or Base Or Both Strong Or Weak Carbonic Acid
Strong And Weak Acids And Bases Acids Are Pretty Basic

Topic 08 Acids Bases 8 3 Strong And Weak Acids And Bases Ppt Download
Why Is Carbonic Acid A Weak Acid Even Though It Gets Completely Dissociated Into H And Co3 Ions Quora
Comments
Post a Comment